Overview
Prostate cancer is the most common cancer and the second leading cause of cancer death in American men. Early diagnosis is essential for better treatment and increasing survival rate. Although the current screening techniques, antigen (PSA) blood testing and digital rectal examination (DRE) are considered sensitive enough for cancer screening, follow-up biopsies have significant shortcomings. Without a good imaging technique to target the needle biopsy in the prostate gland, only about 25% of tests are positive for cancer in more than 1 million prostate biopsies performed each year; the false negative rates range from 25-45% based on the first time biopsy. Although imaging techniques are essential for cancer diagnosis, imaging the structures and lesions within prostates has been a challenging task. The goal of this study is to optimize and evaluate ARFI imaging techniques for prostate imaging.
We have demonstrated that ARFI imaging can clearly portray zonal anatomy and some cancerous lesions in the prostate in ex vivo studies using a linear array. We have transitioned this work into the in vivo setting where were are using a transrectal side-fire array with a custom designed automated rotation stage to obtain 3D ARFI and B-mode image volumes of the prostate, immediately prior to radical prostatectomy surgery. Post-excision, we perform whole-mount histologic processing of the excised prostate to correlate ARFI image findings with histologically confirmed pathology. In our initial in vivo studies, we see similar zonal anatomy and pathology appearance to the ex vivo studies.
We are now using sequences on a state of the art extensively parallel scanner to obtain both ARFI and shear wave sequences concurrently, with the ultimate goal of providing high contrast, high resolution quantitative stiffness image volumes of the entire prostate. These sequences can be used for targeting prostate biopsy, providing image guidance during focal therapy procedures, and to monitor disease progression and response to treatment noninvasively. We are collaborating with Dr. Thomas Polascik in the Department of Surgery (Urology), Dr. John Madden in the Department of Pathology, and Dr. Rajan Gupta in the Department of Radiology on this work.
We are also developing segmentation and registration techniques to align the whole mount histology slides with acquired bmode, ARFI, and MR images. An example of some registered images between whole mount histology, B-mode, ARFI and MR image volumes are shown below.
Image
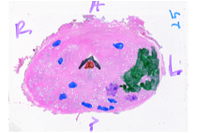
| Image
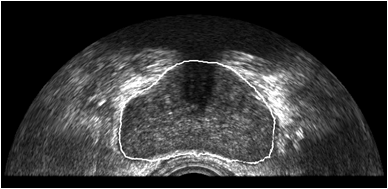
|
Image
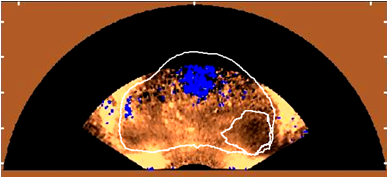
| Image

|
Image
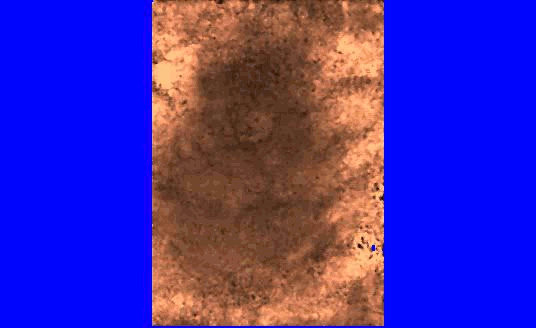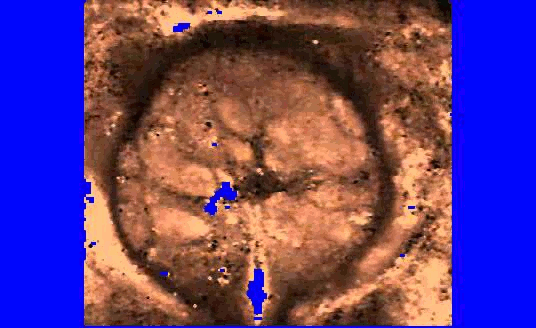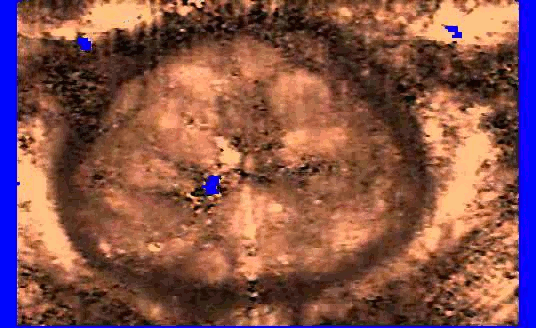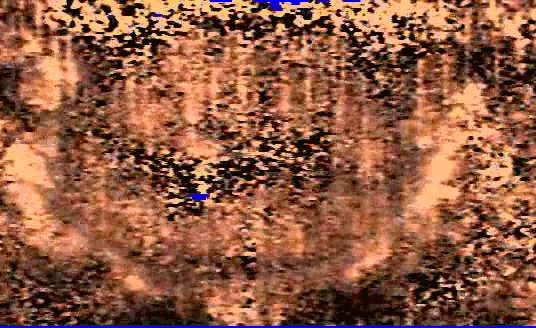
| Image
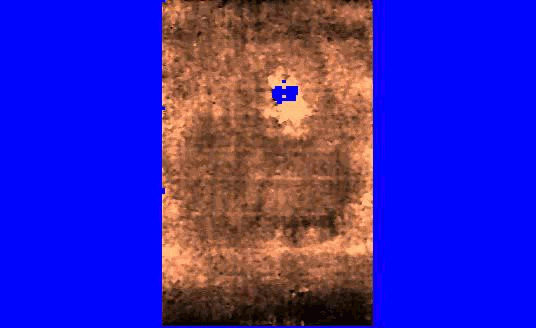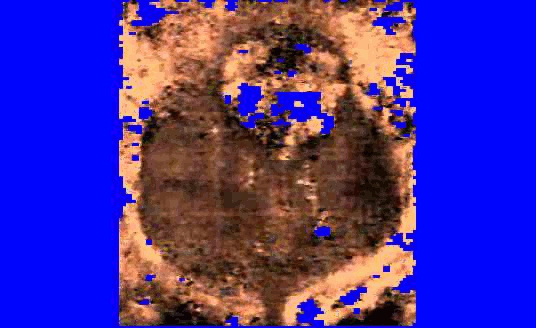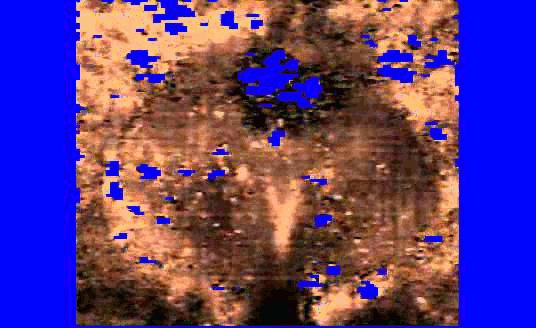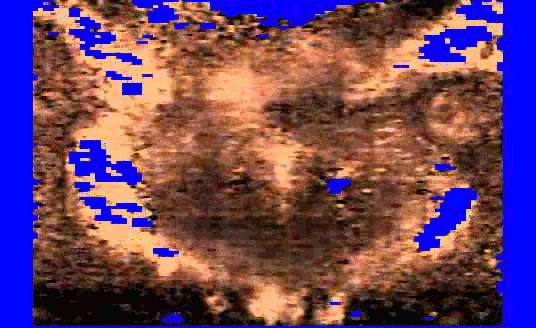
|
Related Publications
- Palmeri et al. "Identifying Clinically Significant Prostate Cancers using 3-D in Vivo Acoustic Radiation Force Impulse Imaging with Whole-Mount Histology Validation" is Open Access avaiable the Ultrasound in Medicine and Biology (http://dx.doi.org/10.1016/j.ultrasmedbio.2016.01.004).
- Palmeri et al. "B-Mode and Acoustic Radiation Force Impulse (ARFI) Imaging of Prostate Zonal Anatomy Comparison with 3T T2-Weighted MR Imaging" (http://uix.sagepub.com/content/37/1/22.short).
- Gupta et al. "Apparent Diffusion Coefficient Values of the Benign Central Zone of the Prostate: Comparison With Low- and High-Grade Prostate Cancer" (http://www.ajronline.org/doi/abs/10.2214/AJR.14.14221).
- Garcia-Reyes et al. "Detection of prostate cancer with multiparametric MRI (mpMRI): effect of dedicated reader education on accuracy and confidence of index and anterior cancer diagnosis" (http://link.springer.com/article/10.1007/s00261-014-0197-7).
- Zhai L, Madden J, Foo W, et al. Acoustic radiation force impulse imaging of human prostate ex vivo. Ultrasound Med. Biol.. 2010;36(4):576–588. (http://www.ncbi.nlm.nih.gov/pubmed/20350685)
- Zhai L, Madden J, Foo WC, Mouraviev V, Polascik T, Palmeri M, Nightingale K. "Characterizing the stiffness of human prostates using acoustic radiation force," Ultrasonic Imaging, 32(4): 201-213, 2010.
- Zhai L, Polascik T, Foo W, Rosenzweig SJ, Palmeri M, Madden, Nightingale KR. “Acoustic Radiation Force Impulse Imaging of Human Prostates: Initial in vivo Results”, Ultrasound Med. Biol., 38 (1):50-61, 2012.
Funding
This work is supported by the National Institutes of Health (R01 CA142824) and DOD PCRP grant W81XWH-16-1-0653.